So it’s the weekend before Christmas and still no sign of snow! The campus is quiet and the labs are pretty much dead. Only a few PhDs and postdocs remain working.
After my time off the week before last I tried to make this final week as productive as I could. I started Monday trying desperately to sort out a reaction from the weekend to no avail at all. I got a disgusting oil that was truly inseparable from starting material despite my best efforts. Two columns and a “whole lotta love” did nothing. I then quickly redesigned one moiety of my molecule, going for a pyrollidine in preference for the piperidine moiety that was in its place. The only difference is one carbon less and I wasn’t exactly optimistic about it, but it was a quick reaction so I figured why not? A colleague of mine even scoffed at the idea that it would give me a higher conversion to product. But you know what… Fuck you @Cormey123 (feel free to drop a tweet with the middle finger emoji, he’ll love it). I made the precursor which crystallised out in a lovely pale green then carried that forward to my next step. The subsequent reaction worked an absolute treat and crystallised out superbly to give pure white crystals. I was elated and surprised at the same time. Below is a .gif (hopefully working) showing you how readily this stuff crashes out.
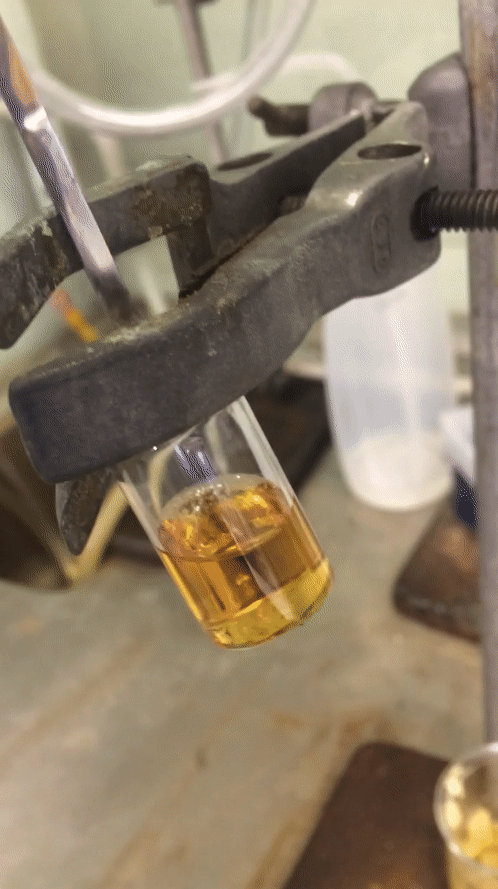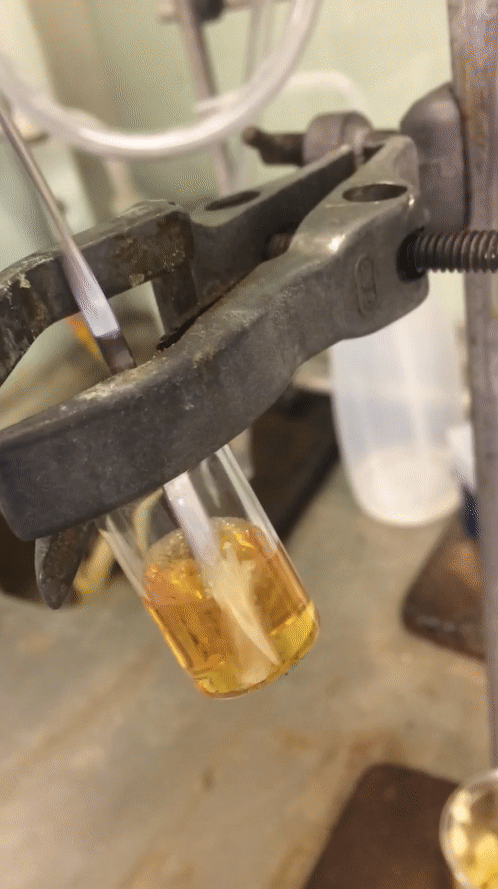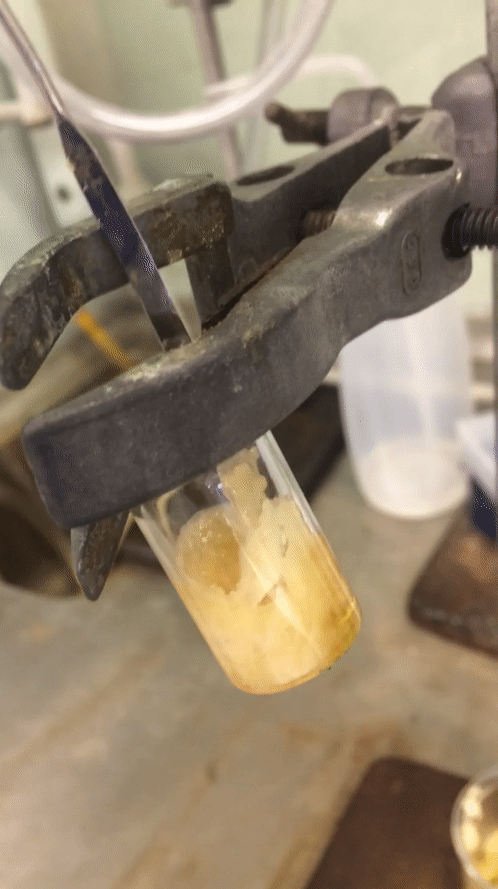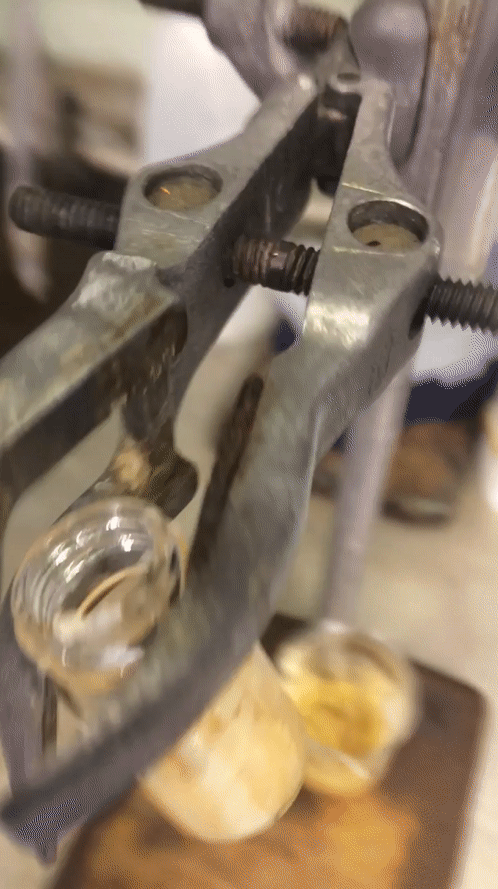
Amazing considering it’s only one carbon less than being a horrible disgusting orange oil full of impurities that do not separate. A quick wash with ice-cold diethyl ether and they were ready to carry forward. So on Wednesday after all my leg work I designed and set up a fairly novel reaction using my lovely crystals.
I did all the prep, I calculated my masses, made sure we had reagents in stock and was geared up for greatness. Obviously I got sod all happen. All that effort and no reaction had occurred. Analysis showed clear starting material only and nothing else of interest. A quick once over by a more seasoned set of eyes confirmed this. I spent much of my Thursday going through this data in an attempt to find something… anything. But no luck. I was down, I was grumpy and I was tired. Then something rather strange and unexpected happened. The penny dropped. I got that funny feeling that others in research will understand. It started slowly at first, soft and gentle. I began by just going through my inventory of chemicals and see what we had in that I might try, noting down stuff that looked possible. Then as my list of potential catalysts grew I became consumed with alternatives, mining the literature for any hint they may be worth a bash. That excitement for research had well and truly grabbed me by balls in a vice like grip, refusing to let me go. I have not had that intense of a feeling since midway through my placement year thinking of alternative electrophiles for my ortho lithiation of diphenylmethane derivatives. Because of the nature of lithiation chemistry and (-)-sparteine catalysis, you cannot just use anything. That, and getting the new molecule to play ball with the chiral shift reagent to prove via NMR that the reaction was enantioselective was a challenge. I have to thank Paddy here and his suggestion of CO2.
Spurred on by my failure I designed a new set of reactions, ten in total.
So on Friday in what can only be described as pissed off defiance, I took to lab and worked late into the night setting up all of my new ideas. Probably none of them will work and that’s ok. Its allowed me the confidence to set up on my own, work in concentrations un-assisted for the first time and be a truly independent researcher for the first time since I started in October. None of my reactions will probably even work. Better for me as it will only make me hungrier for the work. Time now to go and enjoy Christmas, then hammer it harder than ever in the new year. Gone are the low-spirited, sombre thoughts of pure organic chemistry being dead and in their place are high-flying dreams of bio-orthogonal carbon-carbon bond formation. Let’s see what 2017 brings shall we?
This week’s #ReactionRecap is the Gatterman and Gatterman-Koch reaction. Ludwig Gatterman was a German chemist born in 1860. He had a very fearless approach when it came to chemistry it seems which earned him the nickname “der Heros” meaning The Hero for his efforts in the analysis of purified nitrogen trichloride. It is highly explosive and detonated by heat, light and shock. He is also quoted saying “if you are used to handling the substance it is no worse than handling alcohol” in reference to working with hydrocyanic acid! He also noted that the gaseous form of hydrocyanic acid gave a strange taste to his cigars.
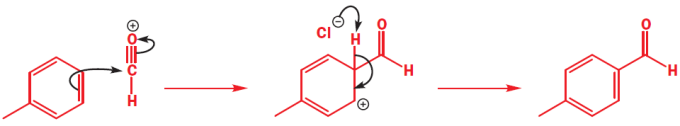
The Gatterman-koch reaction is similar to a Friedal-Crafts and generates aldehydes. It is generated by passing carbon monoxide and HCl through a mixture of the aromatic hydrocarbon, a Lewis acid such as aluminium chloride and a co-catalyst, usually copper (I) chloride. This reaction scope is limited as it does not work with phenolic or amino aromatic species due to complex formation with the Lewis acid. Despite being an old reaction this one carbon electrophile approach is still used heavily in industry for the synthesis of p-tolualdehyde. This mechanism is shown below.
For more reactive aromatic systems such as phenols (but still not amines) a variation of this reaction, called the Gatterman reaction, is employed for preparing aldehydes. Instead of using protonated carbon monoxide, protonated hydrogen cyanide is used (both are isoelectronic). The reaction proceeds through an imine intermediate, ArCH=NH, which under the conditions of the reaction is hydrolysed to the aldehyde.
When such reactive aromatic species as phenols are involved, the Lewis acid does not need to be strong and zinc chloride is often used. As a result of a less reactive system, AlCl3 is required. The zinc chloride can be easily generated from zinc cyanide, Zn(CN)2, and HCl. This also has the advantage of generating the necessary HCN in situ as well.
In a variation of the Gatterman reaction, an alkyl cyanide RCN is used in place of HCN as a method of preparing ketones from reactive aromatic species that do not react well under Friedel–Crafts conditions. The electrophile involved is effectively R–C≡NH+, however the imino chloride, R(C=NH) Cl (analogue of an acyl chloride) is also involved. Similar to the above Gatterman reaction, the imine is again the intermediate. These reactions even work when there are three hydroxyls on the benzene ring!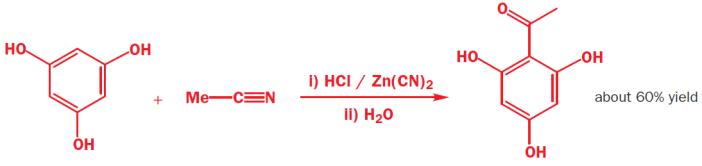
Well that’s me out folks until after Christmas. I’ll still be as prolific as ever on twitter @LewisMGooch trying not start wars with Spanish profs and industry experts as usual. Until the next time have a great xmas, be safe and have fun.